Why are vaccines developed so quickly and treatments so slowly?
In March 2020, the WHO declared COVID-19 a pandemic. Since then, 14 vaccines have entered the global market[1], and the number of immunized people grows every day. Even though vaccine development has never been this fast in history and will save many lives worldwide, people are still getting infected. To save patients afflicted with severe cases of COVID-19 and prevent health care systems from collapsing, effective drug treatments are the next step. But shouldn’t there already be drugs available?
What is the difference between vaccines and drug treatments?
While vaccines protect non-infected people from future infections, drugs help sick people. They alleviate symptoms and shorten the time to recuperate. Vaccines are important to combat a disease in the long run, through reaching herd immunity and protecting risk groups that would otherwise not survive an infection. Drugs, however, are equally important to fight acute infections.
How are vaccines developed?
First, scientists study the pathogen and its way of causing a disease in detail. Researchers then present a part of the virus that can be targeted by a vaccine for further investigation. This first lab stage is called the "research & discovery stage". What follows are pre-clinical trials, which are carried out in animals or cultured cell lines. Here, scientists prove that their vaccine candidate can cause an immune response and is not toxic.
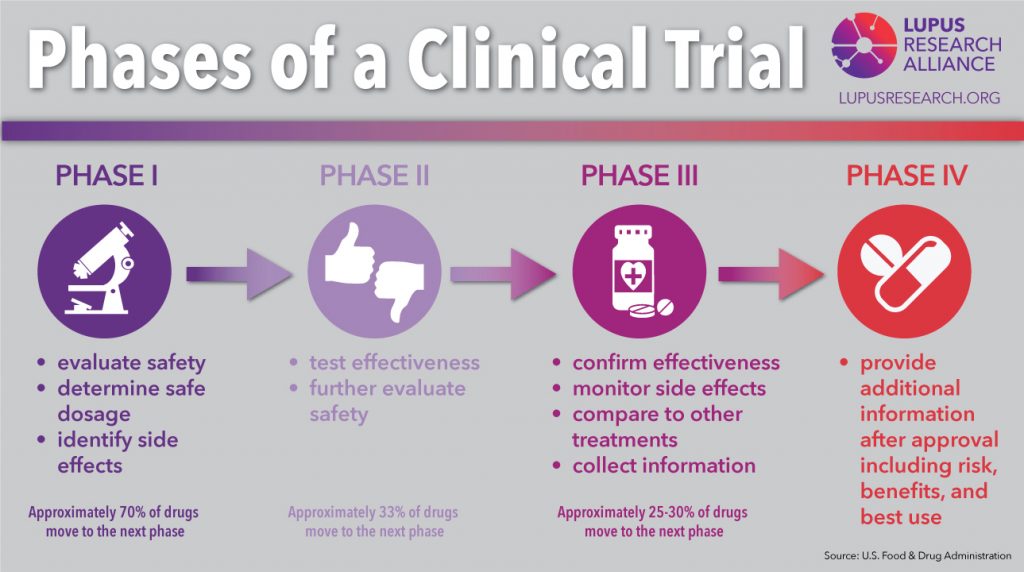
If all data collected until this point looks promising, a clinical trial to test the vaccine in humans follows:
- Phase I examines the safety and dose for the vaccine, as well as the immune response.
- Phase II tests safety, efficacy, dosing, and immune response in a larger and more diverse group of people.
- Phase III tests the vaccine on thousands of subjects to determine efficacy and side effects.
- If successful, the vaccine is approved by the FDA in the US or the EMA in the EU. After approval, the vaccine is monitored in the real world, and more data is collected.
How come it progressed so fast for the coronavirus?
On January 10th, 2020, the genome of SARS-CoV-2 was published by a consortium of Chinese and Australian scientists[2]. This started global efforts to develop vaccines. For the first time, corporations, governments, and scientists from academia worked together on this scale to end the pandemic. The first vaccine approved in the western world was Pfizer-BioNTech in December 2020 (approved in the EU[3] and the US[4]). It took eleven months, a record time never reached for any other vaccine in history.
The fastest vaccine developed before COVID-19 was the mumps vaccine in the 1960s[5]. It took only four years to develop, which is amazingly fast compared to the average 10 years it takes for a vaccine to progress from basic research to approval[6]. Given this info, how was it possible to get SARS-CoV-2 vaccines ready in less than a year?
First, SARS-CoV-2 did not come out of nowhere, entirely. For years scientists have been studying its relatives SARS and MERS, their way of infecting cells, their proteins, and genetics[7]. Because of that, scientists did not have to start from scratch with researching these matters for the new coronavirus. Of course, there are differences, but the viruses’ general framework shows many similarities.
The cost of developing a vaccine, on average, surpasses the 1-billion-dollar mark[8], with a lot of the money spent on candidates that turn out to be failures. For many infectious diseases, funding simply cannot be sustained throughout development, especially if the disease is rare or occurs only locally. This was not a problem for the development of SARS-CoV-2 vaccines. Massive funding from governments and companies gave scientists more than enough resources to test their vaccine candidates. Also, because of all these resources, the developers were able to run several stages of testing in parallel[9].
Probably the most interesting reason for having vaccines this fast are the new vaccine technologies that have been developed since the turn of the century: mRNA and vector vaccines. Scientists often refer to them as vaccine platforms because they are technologies into which you only need to insert the part that is specific to a virus. For this, you need the virus’ genetic information, which was published early in this pandemic. Vaccine developers then inserted SARS-CoV-2 into their systems and were ale to quickly procede with the trials.
In the past, inactivated viruses or isolated viral proteins were used in vaccines. These, however, are highly specific to the virus you are trying to fight. This means, it was necessary to start at a basic level again every time a new vaccine was developed. Researchers hope that through these new vaccine platforms, the time for vaccine development in general will be a lot shorter in the future.
How is drug development different and why does it take so long?
The development of a new drug takes years, too. Generally, the timelines for drug and vaccine development show similar steps: Research & discovery, pre-clinical and clinical trials, approval and monitoring also apply for drug development.
Reaching the approval of an effective drug costs around $1,335.9 million[10], and failures are possible at every step of the way—like promising candidates from the pre-clinical stages showing no effect in humans, etc.
However, when comparing modern drug development to the new vaccine platforms, we can see just how much more complicated it is. Antiviral drugs are molecules that interact with parts of the virus and block it from entering a cell, stop it from replicating or stop another vital step of the infection path. There are also drugs that interact with parts of our own immune system to stop the disease from escalating.
Structurally, a drug candidate has to exactly fit its target, which is often a protein. This triggers two problems: What target should be chosen and what should the drug molecule look like.
Nowadays, millions of molecules are screened for their interaction with viral targets. This is done through computerized models but still takes a lot of time. Once there is a lead structure—a potentially effective molecule—, it is tested in pre-clinical trials and optimized structurally. During optimization, many hundreds of similar molecules are compared to see if their properties improve.
Different approaches to develop a drug take different amounts of time. One shortcut to developing an effective treatment is the repurposing of an already approved drug or a shelved candidate. The second fastest way is to develop a therapeutic antibody, followed by classic screening for a new drug.
Repurposing drugs for new diseases
Reusing drugs or drug candidates that have already been evaluated for safety greatly shortens the development time. It is even better when an already approved drug or a drug that is already backed with significant data from human trials shows an effect on the new disease.
An example for this is the first HIV medication AZT. It was first developed in 1964 as a potential cancer therapy, but later was found not to be very effective. In the 1980s, it was included in a screening for AIDS treatment and was found to interfere with HIV’s replication. It was later shown to decrease the death rate in people with AIDS and subsequently approved for treatment[11].
Remdesivir—a dead end?
Remdesivir is an antiviral drug that was designed to interfere with the replication of the genome of RNA-based viruses. The drug was first developed as a potential treatment for hepatitis C and respiratory syncytial virus and was later tested against the Ebola virus[12], which did not lead to convincing results[13].
But what do COVID-19 studies with remdesivir say?
The EU and US approved this medication in 2020 based on trials with patients that had moderate or severe cases. One study found that hospitalized patients with moderate COVID-19 benefited from a 5-day treatment with remdesivir.[14] For severe cases, there is some evidence that remdesivir could shorten the time to recuperate better than a placebo[15].
But the WHO is now advising against using the drug based on a meta-analysis they did. This convinced the EMA to re-evaluate remdesivir and maybe even take back the approval in the EU[16]. In the WHO study, the effect of four repurposed COVID-19 treatments—including remdesivir—on 11,330 adults was examined[17]. In this analysis, remdesivir showed little to no effect on hospitalized patients with COVID-19, as indicated by overall mortality, need of artificial ventilation, and length of hospital stay.
The other repurposed drugs from the WHO analysis were hydroxychloroquine (a treatment used against malaria), Lopinavir (a protease inhibitor used against HIV), and interferon beta-1a (an immune modulating drug for MS treatment). None of these showed a beneficial effect against acute COVID-19.
Dexamethasone is a success
The most dangerous aspect of a COVID-19 infection is that the person’s immune system overreacts and attacks the body in addition to the virus. Steroids are often used to dampen an immune response, so the commonly available drug dexamethasone was tried as a treatment. This has proven to be highly effective and has now become part of the standard care for COVID-19 patients[18].
Other drugs currently under evaluation
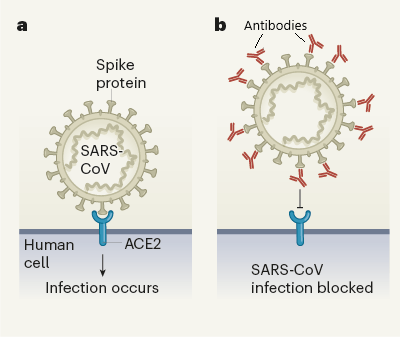
Monoclonal antibody treatments are currently under review in the EU and US.[19] Their effect: When the antibody attaches to the spike protein, the virus cannot enter the body’s cells, depriving it of its means of replication. Some of these treatments are combinations of two different antibodies that can attach to different parts of the spike, in theory, binding it more effectively.
The three treatments currently in the EMA review pipeline are: bamlanivimab and etesevimab, REGN-COV2[20], and regdanvimab[21]. The FDA[22] has authorized several monoclonal antibodies for emergency use and they have been shown to be effective at reducing the symptoms of COVID-19 if administrated early in the course of the disease[23].
Ongoing efforts
As there is more and more structural data available on SARS-CoV-2’s proteins, there are also more interaction studies with potential drug molecules and combinations.
Still, drug development takes time. In comparison to vaccines, which have just had a technology revolution, drugs will take longer from basic research to being ready for use. But there are already many different types of drugs under development, since funding is not a problem for COVID-19 treatments, and international collaboration speeds up the process as well.
Whether any of the current candidates will prove effective in treating COVID-19, remains to be seen. Maybe even a combined therapy with multiple drugs could be used to achieve the desired outcome.
[1] https://www.unicef.org/supply/covid-19-vaccine-market-dashboard
[2] https://virological.org/t/novel-2019-coronavirus-genome/319
[3] https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty
[4] https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
[5] Tulchinsky, Theodore H.. “Maurice Hilleman: Creator of Vaccines That Changed the World.” Case Studies in Public Health (2018): 443–470. doi:10.1016/B978-0-12-804571-8.00003-2
[6]COVID-19 vaccine development pipeline gears up. Mullard, Asher. The Lancet, Volume 395, Issue 10239, 1751 - 1752
[7] Abdelrahman Zeinab, Li Mengyuan, Wang Xiaosheng. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Frontiers in Immunology 11 (2020). doi:10.3389/fimmu.2020.552909
[8]Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study, Gouglas, Dimitrios et al. The Lancet Global Health, Volume 6, Issue 12, e1386 - e1396
[9] https://www.nature.com/articles/d41586-020-03626-1
[10] Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323(9):844–853. doi:10.1001/jama.2020.1166
[11] https://www.niaid.nih.gov/diseases-conditions/antiretroviral-drug-development
[12] https://www.gilead.com/-/media/gilead-corporate/files/pdfs/covid-19/gilead_rdv-development-fact-sheet-2020.pdf
[13] Pardo, Joe et al. “The journey of remdesivir: from Ebola to COVID-19.” Drugs in context vol. 9 2020-4-14. 22 May. 2020, doi:10.7573/dic.2020-4-14
[14] https://jamanetwork.com/journals/jama/fullarticle/2769871
[15] https://www.nejm.org/doi/10.1056/NEJMoa2007764
[16] https://www.ema.europa.eu/en/news/update-remdesivir-ema-will-evaluate-new-data-solidarity-trial
[17] Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. 384, 497-511 (2020).
[18] https://www.covid19treatmentguidelines.nih.gov/immunomodulators/corticosteroids/
[19] https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-under-evaluation
[20] https://www.ema.europa.eu/en/news/ema-starts-rolling-review-regn-cov2-antibody-combination-casirivimab-imdevimab
[21] https://www.ema.europa.eu/en/news/ema-starts-rolling-review-celltrion-antibody-regdanvimab-covid-19
[22] https://www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19
[23] https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-
