Introduction:
Vaccination is a great means of achieving public protection against diseases. The main goal of any vaccine manufacturer is to produce a vaccine that will be safe and effective in preventing the target disease. Before any vaccine is rolled out for mass vaccination or campaign, it must have met the required rigorous scientific and ethical standards, ensuring safety, efficacy, purity, and potency (1). A vaccine is considered safe and effective when used correctly, leading to a successful vaccination campaign. However, from the general health point-of-view, vaccines are not risk-free and there are occasionally Adverse Events Following Immunization (AEFI) (2). The risk is of course outweighed by the benefits of vaccines as they protect us from vaccine-preventable diseases. Here, I will discuss the safety measures vaccination undergoes for it to be a success.
What does vaccination mean?
Vaccination is a simple, safe, and effective way of protecting people against harmful diseases, before they come into contact with them (3).
What is vaccination safety?
This entails the absence of preventable harm to the recipient, health care worker and the society at large during and after vaccination.
What are the stages of vaccine development before vaccination?
Vaccine development starts with the assessment of public health needs and priorities which progresses to scientific research on the target disease, pre-clinical and eventually, clinical trials (4). The following are the stages of vaccine development:
Research stage; involves the identification and isolation of the target antigen.
Pre-clinical stage; the potential vaccine is being tested on cells and animals after a careful examination.
Clinical trials (three phases); if the vaccine is safe in animals, it will then be suggested to be tested in human volunteers.
The clinical trials are usually in three phases.
- Phase 1; trials involve a smaller number of people of about twenty to hundred healthy volunteers, this is designed to test and determine vaccine safety, doses, immune response (immunogenicity) and route of administration.
- Phase 2; starts after the successful completion of phase 1. This may involve hundreds of volunteers with double-blind (the researchers and the research volunteers do not know if the research volunteers are in the vaccine group or the placebo group, to prevent bias) studies with a placebo-control group. It further tests safety and the amount of dose to provoke an immune response.
- Phase 3; hundreds to thousands of volunteers are given the vaccine in comparison with the placebo group. It involves a randomized double or single-blind (the research volunteers do not know whether they are in the vaccine group or the placebo group, to prevent bias), placebo-controlled group (inactive substance, sometimes another type of vaccine is given to the research volunteers) to evaluate more on the vaccine safety, efficacy and side effects.
This is then followed by regulatory review, approval and manufacturing. After the product is rolled out, a surveillance system is established for continuous monitoring of the vaccine, immunization coverage and possible adverse reactions for risk-management (4,5). COVID-19 vaccines underwent these processes as well.
Who is responsible for vaccine approval?
Following scientific, ethical and international standards, approval on clinical trials, results, and licensing are done by the national and regional regulatory authorities in the countries where vaccines are manufactured (6). This must be guided by principles of fairness, transparency and accountability to ensure safety.
How are vaccines stored and handled?
Cold chain, sometimes referred to as “Supply chain” is the system used for storing vaccines in good condition. In the cold chain system, the levels designed to keep vaccines within recommended temperature ranges, from the point of manufacture to the point of administration differ. This means that the storage temperature range for every level may differ provided the potency of the vaccine is maintained. Vaccines can be sensitive to freezing or light but all vaccines are sensitive to heat. This is what the Vaccine Vial Monitor (VVM) on the vial helps to monitor. VVM is a chemical indicator label attached to the vaccine container (vial and ampoule) by the vaccine manufacturer which helps to monitor the temperature of a vaccine and ensure that no heat-damaged vaccine is administered. If the colour of the inner square is the same colour or darker than the outer circle, the vaccine has been exposed to too much heat and should be discarded. All vaccines must be discarded after the expiry date or six hours of opening. The recommended diluent is used for the reconstitution of freeze-dried vaccines before use.
Are some vaccines made for some persons?
Vaccination is carried out based on dosage, age group or sex. This is because there is a specific group of people used during the clinical trials who will determine the first target group to be vaccinated when the vaccine is rolled out. Notwithstanding, other group of people may still receive the vaccine as the vaccination goes on once safety is ensured. Please always check the fact sheet for any vaccine you wish to receive on the manufacturer`s website for such updates.
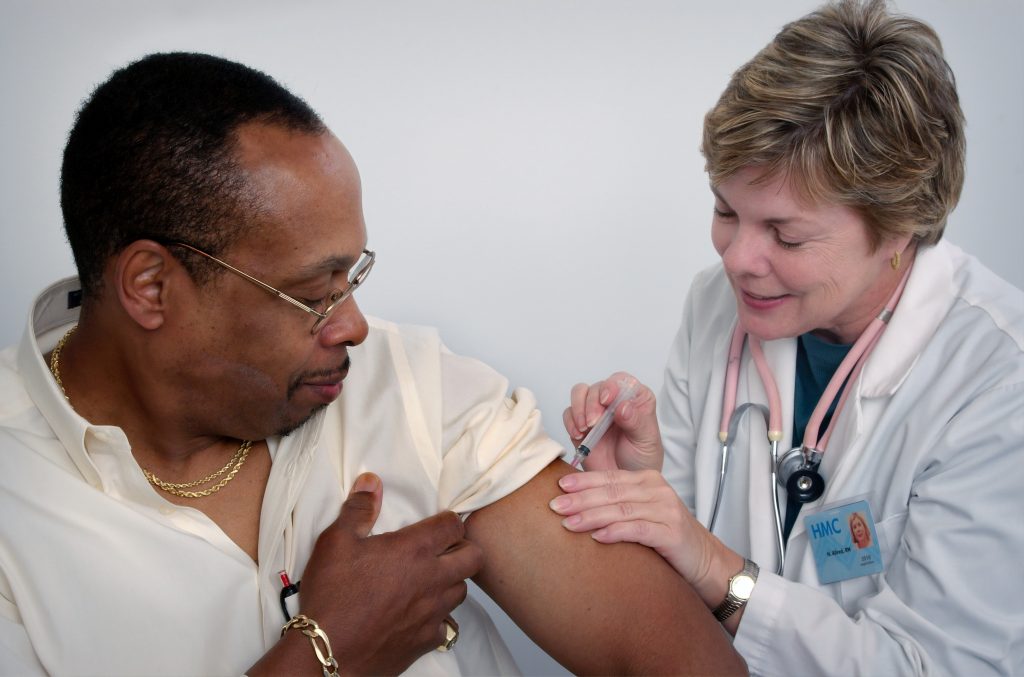
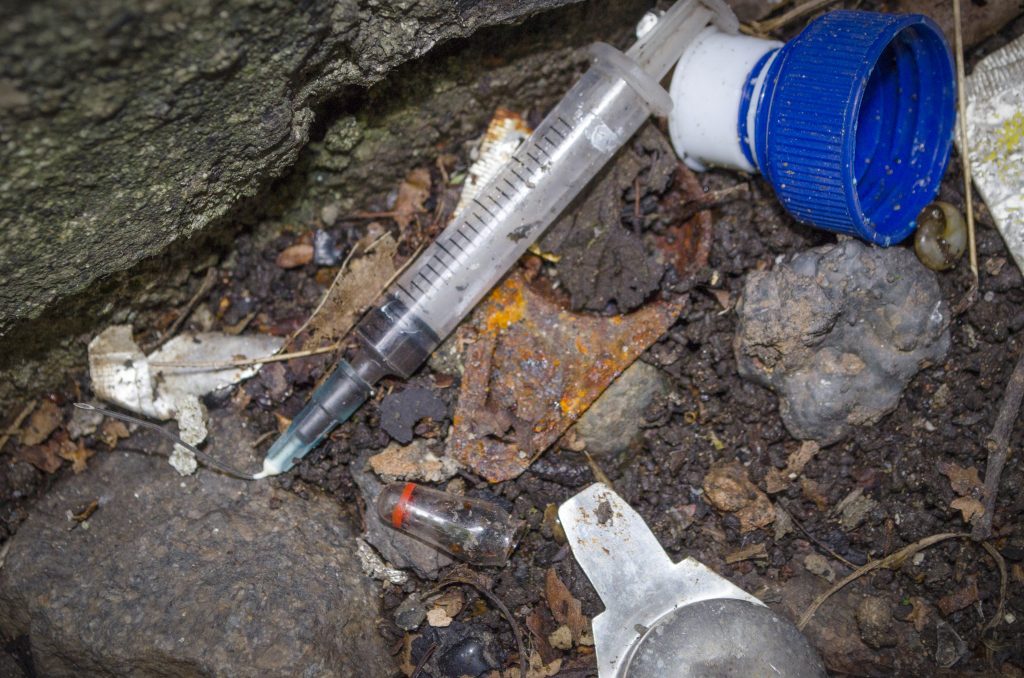
How are injection devices disposed of after a vaccination session?
Injection equipment can pose danger to the environment if not well disposed of, leading to environmental pollution. When needles and syringes are thrown into the bodies of water, they cause contamination to the environment and injury to the wildlife. Safety boxes are sharps waste containers that needles cannot penetrate, they are used to keep the used needles and syringes temporarily during a vaccination session. It is disposed of immediately after vaccination sessions to maintain safety. Appropriate use of a safety box is necessary to avoid needle-stick injuries (when the skin is pricked by needles accidentally) to the health care worker or other individuals. Special incinerators are used to burn these needles and syringes to minimise the toxic release into the environment.
Source: Adobe Stock

Source: Adobe Stock
What is Adverse Events Following Immunization (AEFI)?
This is any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. AEFI can be vaccine product-related, vaccine quality defect-related, vaccination error- related, vaccination anxiety-related or a coincidental event.
Some minor vaccine reactions-AEFIs, you need to know:
- Pain, redness, swelling at the site of injection
- Irritability, malaise
- Slight fever
- Slight headache
- Mild muscle pain
- Loss of appetite
- Joint pain
- Lymphadenopathy (swollen lymph nodes, it can be under the armpit in the same arm of injection, etc.)
Some rare and severe vaccine reactions-AEFIs, you need to know:
- Febrile seizures (convulsions that occur usually in children as a result of high body temperature)
- Thrombocytopenia (low platelet count in the blood which usually helps to stop bleeding when one gets injured)
- Anaphylaxis (severe allergic reaction)
- Sterile abscess (swelling in the injection site usually occurs when an injection is not completely absorbed into the skin which makes pus build up in the tissue)
- Difficulty in breathing
- Encephalopathy (damage to the brain, affecting one`s mental state)
- Persistent inconsolable crying or screaming
Severe AEFIs are rare, however, every AEFI must be reported to the health care worker or appropriate authority.
Before receiving any vaccine, please discuss with the vaccination provider about all of your medical conditions such as:
- past and present allergic reactions
- current fever
- if you are taking blood thinner medications
- if you have any bleeding disorder
- if you are immunocompromised
- if you are on a medication that affects your immune system
- if you are pregnant or plan to become pregnant very soon
- if you are breastfeeding
- your previous vaccination history (7,8).
What does surveillance (pharmacovigilance) mean?
Pharmacovigilance is a part of the surveillance system designed to detect, assess, understand, respond and preventing adverse drug reactions, including reactions to vaccines-AEFIs in every country. Both national and international levels have surveillance systems for good monitoring and immediate actions in response to AEFIs. This helps to ensure the safety of vaccines even as vaccination is ongoing (2).
Conclusion:
Vaccines follow many rigorous scientific processes before and after being approved to ensure safety and effectiveness. Vaccination is safe when no harm is posed to the patient, health worker and society (avoiding pollution and injuries through proper disposal of injection wastes). If a vaccine requires a subsequent dose, you must receive the same type of vaccine as the initial dose. Always keep your vaccination card for the next visit and follow your vaccination schedule. It is encouraged to not go for a second dose of vaccine if you had a serious allergic reaction or side effect after the first dose. Discuss your medical history with the vaccination provider before taking a vaccine. Please wait for some time after receiving your vaccine for a little safety monitoring before going home. Vaccines work!
References:
1. Centers for Disease Control and Prevention. U.S. Vaccine Safety - Overview, History, and How It Works | CDC [Internet]. 2020 [cited 2021 Mar 4]. Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/history/index.html
2. World Health Organization. WHO Vaccine Safety Basics [Internet]. [cited 2021 Mar 3]. Available from: https://vaccine-safety-training.org/overview-and-outcomes-1.html
3. Vaccination_World Health Organization. Vaccines and immunization: What is vaccination? [Internet]. [cited 2021 Mar 3]. Available from: https://www.who.int/news-room/q-a-detail/vaccines-and-immunization-what-is-vaccination
4. Centers for Disease Control and Prevention. Ensuring Vaccine Safety | CDC [Internet]. 2020 [cited 2021 Mar 3]. Available from: https://www.cdc.gov/vaccinesafety/ensuringsafety/index.html
5. Mitchell VS, Philipose NM, Sanford JP. Stages of Vaccine Development_Institute of Medicine (US) Committee on the Children’s Vaccine Initiative: Planning Alternative [Internet]. The Children’s Vaccine Initiative: Achieving the Vision. National Academies Press (US); 1993 [cited 2021 Mar 3]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK236428/
6. World Health Organization_ Vaccine Safety. Vaccines and immunization: Vaccine safety [Internet]. [cited 2021 Mar 3]. Available from: https://www.who.int/news-room/q-a-detail/vaccines-and-immunization-vaccine-safety
7. Moderna. Emergency Use Authorization (EUA) | Moderna COVID-19 Vaccine [Internet]. https://www.modernatx.com/. [cited 2021 Mar 16]. Available from: https://www.modernatx.com/covid19vaccine-eua/
8. Pfizer-BioNTech. Pfizer-BioNTech COVID-19 Vaccine | cvdvaccine.com [Internet]. [cited 2021 Mar 16]. Available from: https://www.cvdvaccine.com
